Have you ever thought about how pressure and density impact our lives? From morning to night, they play a significant role.
Interestingly, in our daily routines, we rarely consider density. But, Scuba Diving will change that! This article explores the relationship of pressure and density of a gas such as air, making it easy for everybody to understand. So let’s dive in!
The Connection Between Pressure and Density
We’ve already discussed how pressure variations affect volume in this article.
To review, as pressure increases, the density of a gas in a flexible container, your lung, increases proportionally at the same volume, 2 X the pressure = 2 X the density at the same volume, your lungs. This is also known as Boyle’s Law.
Now, let’s take a fresh look at that concept.
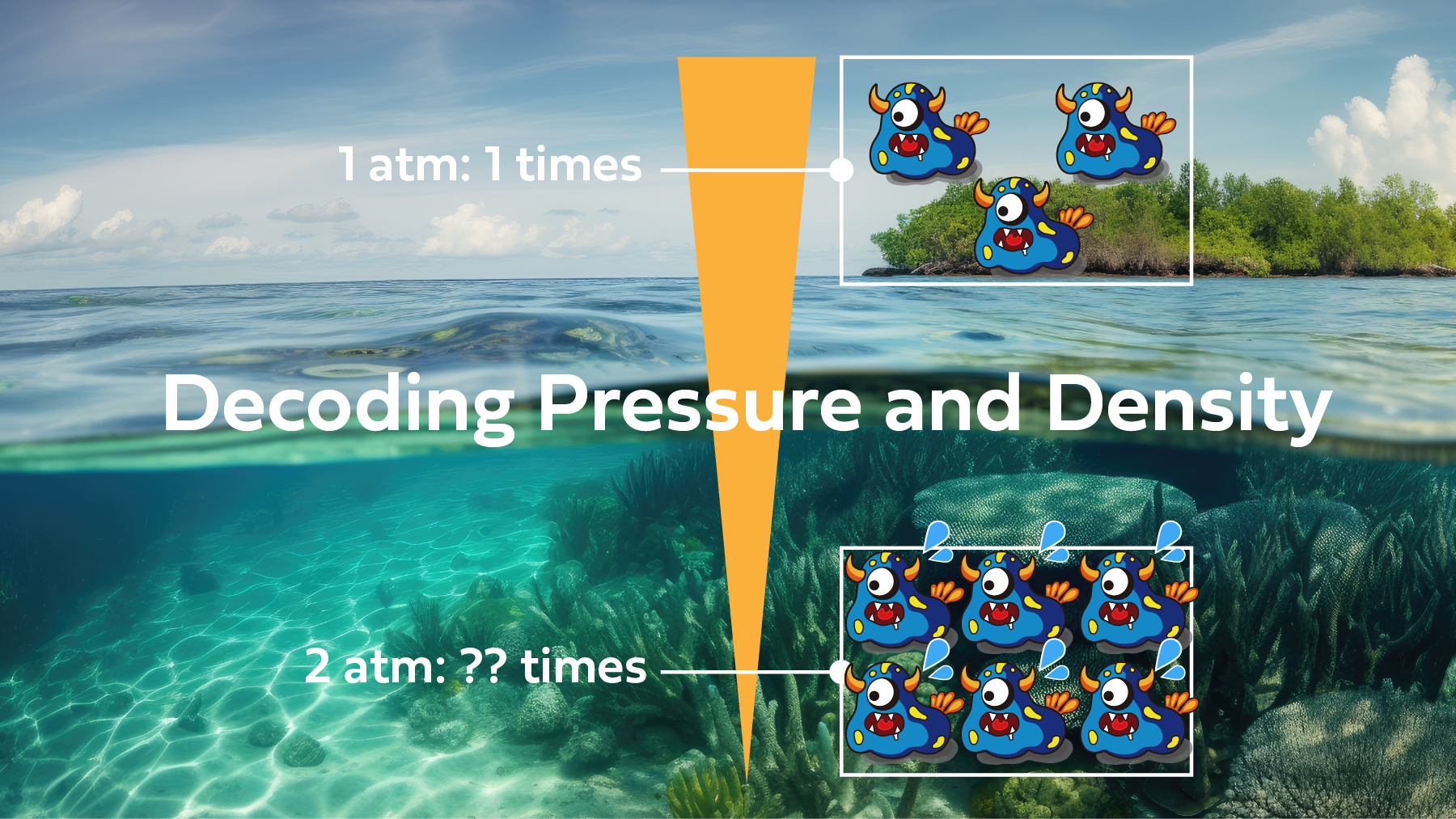
If we think of air as containing X number molecules, then as the pressure increases, they come closer together, increased density, 2X at 10 Meters (33’) However, before and after the change, the total number of molecules remains the same.
Yet, upon closer reflection, you’ll realize that within the same space (volume), your lungs, the number of molecules has doubled with double the pressure.
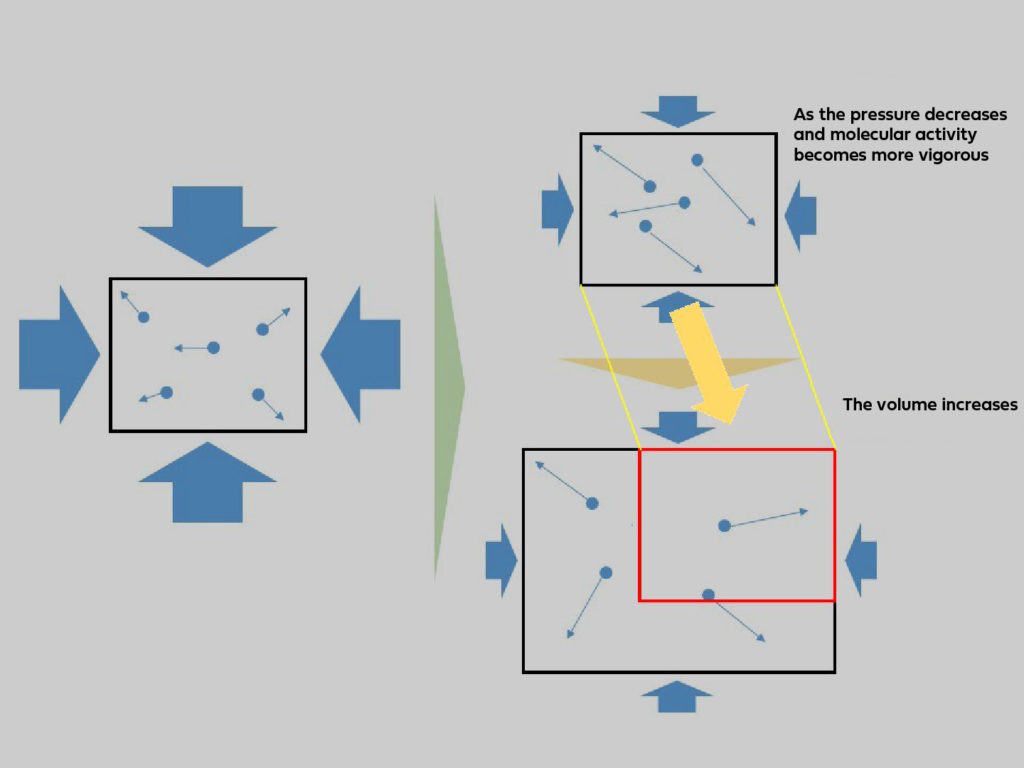
When pressure decreases, density also decreases.
That’s right, pressure and density are proportional to each other!
The quantity of molecules per constant unit volume, your lungs, has decreased.
If pressure doubles, the density also doubles, 10 Meters (33′) 2X Molecules for same volume.
If pressure triples, the density triples, 20 Meters (66′) 3X Molecules for same volume.
The Relationship Between Air Consumption and Depth
Now let’s explore how changes in density affect your air consumption while diving.
When you inhale air from a scuba tank (cylinder) underwater, the volume of each breath remains constant. But have you ever wondered how the pressure of the air you breathe changes with depth?
Imagine you have a tank with 100 air particles inside. When you take a breath at the surface (1 atmosphere of pressure), you inhale one air particle.
Now, let’s consider what happens when you dive to a depth of 10 meters.
Do you think you’ll still inhale one particle? Well, not quite…
As we mentioned earlier, with increasing pressure, the density of air particles also increases. This means that at 10 meters, the same volume that before contained one particle now contains two particles.
So, with each breath, you’ll be inhaling two air particles instead of one!
Pretty much as described in this image:
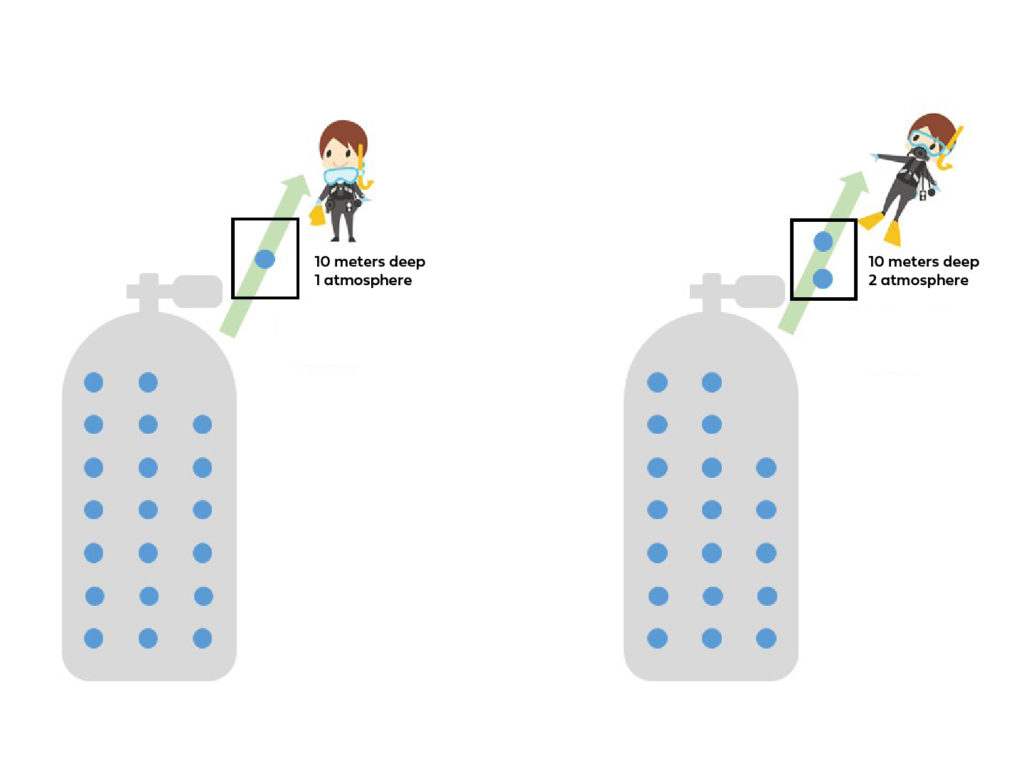
You can see that with the same tank (cylinder) you can breathe 100 times on the surface but only 50 times at a 10m depth.
To put it simply, the deeper you dive, the higher the water pressure and air density become. Consequently, you consume more air with each breath. it is vitally important to remember that the deeper you dive the less air you have available.
This has implications for your dive time and the amount of air remaining in your tank (cylinder). When diving deeper, your air supply will deplete faster, which is why it’s crucial to check your remaining air pressure more often.
So, there is not a simple answer when someone asks you how long you can dive with one tank (cylinder). The answer depends on various factors, including the depth and your consumption rate which can vary under different circumstances. If you’re relaxed, you have a low consumption rate but if stressed it can increase by as much as 20 times. Always surface with at least 30 Bar/500 PSI reserve left in your cylinder.
Your recreational depth limit is 20 Meters or 60′, to go deeper it’s highly recommended to take a deep diving class due to increased air consumption and nitrogen in-gassing.
Remember to plan your dives accordingly and always keep track of your air supply to ensure a safe and enjoyable diving experience!






